As manufacturing to high-end transformation, the rapid development in the field of clean energy and semiconductor and photovoltaic industry development, with high efficiency and high precision processing ability of diamond tools growing demand, but artificial diamond powder as the most important raw material, diamond county and the matrix holding force is not strong easy early carbide tool life is not long. In order to solve these problems, the industry generally adopts the diamond powder surface coating with metal materials, to improve its surface characteristics, enhance durability, so as to improve the overall quality of the tool.
The diamond powder surface coating method is more, including chemical plating, electroplating, magnetron sputtering plating, vacuum evaporation plating, hot burst reaction, etc., including chemical plating and plating with mature process, uniform coating, can accurately control the coating composition and thickness, the advantages of customized coating, has become the industry two most commonly used technology.
1. chemical plating
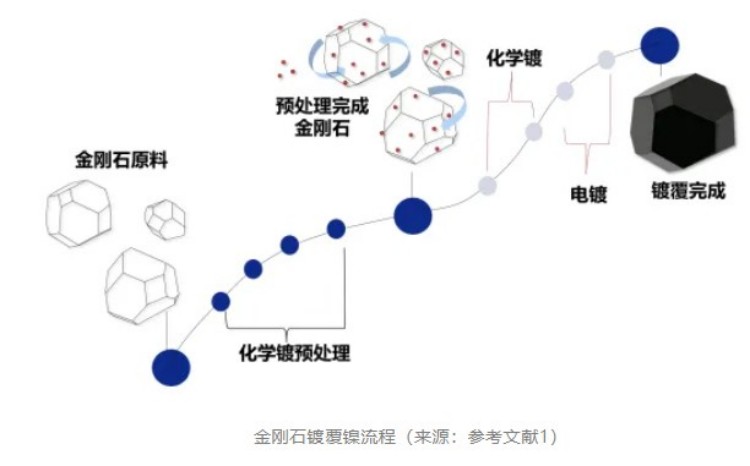
Diamond powder chemical coating is to put the treated diamond powder into the chemical coating solution, and deposit the metal ions in the coating solution through the action of the reducing agent in the chemical coating solution, forming a dense metal coating. At present, the most widely used diamond chemical plating is chemical nickel plating-phosphorus (Ni-P) binary alloy is usually called chemical nickel plating.
01 Composition of chemical nickel plating solution
The composition of chemical plating solution has a decisive influence on the smooth progress, stability and coating quality of its chemical reaction. It usually contains main salt, reducing agent, complexer, buffer, stabilizer, accelerator, surfactant and other components. The proportion of each component needs to be carefully adjusted to achieve the best coating effect.
1, main salt: usually nickel sulfate, nickel chloride, nickel amino sulfonic acid, nickel carbonate, etc., its main role is to provide nickel source.
2. Reductive agent: it mainly provides atomic hydrogen, reduces Ni2 + in the plating solution into Ni and deposits it on the surface of diamond particles, which is the most important component in the plating solution. In the industry, sodium secondary phosphate with strong reduction ability, low cost and good plating stability is mainly used as the reducing agent. The reduction system can achieve chemical plating at low temperature and high temperature.
3, complex agent: the coating solution can precipitate precipitation, enhance the stability of the coating solution, extend the service life of the plating solution, improve the deposition speed of nickel, improve the quality of the coating layer, generally use succinin acid, citric acid, lactic acid and other organic acids and their salts.
4. Other components: the stabilizer can inhibit the decomposition of the plating solution, but because it will affect the occurrence of chemical plating reaction, need moderate use; the buffer can produce H + during the chemical nickel plating reaction to ensure the continuous stability of pH; the surfactant can reduce the coating porosity.
02 The chemical nickel-plating process
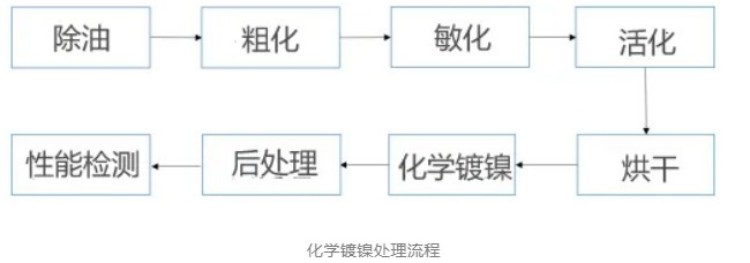
The chemical plating of sodium hypophosphate system requires that the matrix must have certain catalytic activity, and the diamond surface itself does not have catalytic activity center, so it needs to be pretreated before the chemical plating of diamond powder. The traditional pretreatment method of chemical plating is oil removal, coarsening, sensitization and activation.
(1) Oil removal, coarsening: oil removal is mainly to remove the oil, stains and other organic pollutants on the surface of the diamond powder, to ensure the close fit and good performance of the subsequent coating. The coarsening can form some small pits and cracks on the surface of diamond, increase the surface roughness of diamond, which is not only conducive to the adsorption of metal ions in this place, facilitate the subsequent chemical plating and electroplating, but also form steps on the surface of diamond, providing favorable conditions for the growth of chemical plating or electroplating metal deposition layer.
Usually, the oil removal step usually takes the NaOH and other alkaline solution as the oil removal solution, and for the coarsening step, the nitric acid and other acid solution is used as the crude chemical solution to etch the diamond surface. In addition, these two links should be used with ultrasonic cleaning machine, which is conducive to improving the efficiency of diamond powder oil removal and coarsening, save the time in the oil removal and coarsening process, and ensure the effect of oil removal and coarse talk,
(2) Sensitization and activation: the sensitization and activation process is the most critical step in the whole chemical plating process, which is directly related to whether the chemical plating can be carried out. Sensitization is to adsorb easily oxidized substances on the surface of diamond powder which does not have autocatalytic ability. The activation is to adsorb the oxidation of hypophosphoric acid and catalytically active metal ions (such as metal palladium) on the reduction of nickel particles, so as to accelerate the deposition rate of coating on the surface of diamond powder.
Generally speaking, the sensitization and activation treatment time is too short, the diamond surface metal palladium point formation is less, the adsorption of the coating is insufficient, the coating layer is easy to fall off or difficult to form a complete coating, and the treatment time is too long, will cause the palladium point point waste, therefore, the best time for sensitization and activation treatment is 20~30min.
(3) Chemical nickel plating: the chemical nickel plating process is not only affected by the composition of the coating solution, but also affected by the coating solution temperature and PH value. Traditional high temperature chemical nickel plating, the general temperature will be in 80~85℃, more than 85℃ easy to cause the decomposition of the plating solution, and at the lower than 85℃ temperature, the faster the reaction rate. On PH value, as the pH increase coating deposition rate will rise, but the pH will also cause nickel salt sediment formation inhibit chemical reaction rate, so in the process of chemical nickel plating by optimizing the chemical plating solution composition and ratio, chemical plating process conditions, control the chemical coating deposition rate, coating density, coating corrosion resistance, coating density method, coating diamond powder to meet the demand of industrial development.
In addition, a single coating may not achieve the ideal coating thickness, and there may be bubbles, pinholes and other defects, so multiple coating can be taken to improve the quality of the coating and increase the dispersion of coated diamond powder.
2. electro nickelling
Due to the presence of phosphorus in the coating layer after diamond chemical nickel plating, it leads to poor electrical conductivity, which affects the sand loading process of the diamond tool (the process of fixing the diamond particles on the matrix surface), so the plating layer without phosphorus can be used in the way of nickel plating. The specific operation is to put the diamond powder into the coating solution containing nickel ions, diamond particles contact with the power negative electrode into the cathode, nickel metal block immersed in the plating solution and connected with the power positive electrode to become the anode, through the electrolytic action, the free nickel ions in the coating solution are reduced to atoms on the diamond surface, and the atoms grow into the coating.
01 Composition of the plating solution
Like the chemical plating solution, the electroplating solution mainly provides the necessary metal ions for the electroplating process, and controls the nickel deposition process to obtain the required metal coating. Its main components include main salt, anode active agent, buffer agent, additives and so on.
(1) Main salt: mainly using nickel sulfate, nickel amino sulfonate, etc. Generally, the higher the main salt concentration, the faster the diffusion in the plating solution, the higher the current efficiency, the metal deposition rate, but the coating grains will become coarse, and the decrease of the main salt concentration, the worse conductivity of the coating, and difficult to control.
(2) Anode active agent: because the anode is easy to passivation, easy to poor conductivity, affecting the uniformity of current distribution, so it is necessary to add nickel chloride, sodium chloride and other agents as anodic activator to promote anode activation, improve the current density of the anode passivation.
(3) Buffer agent: like the chemical plating solution, the buffer agent can maintain the relative stability of the plating solution and the cathode pH, so that it can fluctuate within the allowable range of the electroplating process. Common buffer agent has boric acid, acetic acid, sodium bicarbonate and so on.
(4) Other additives: according to the requirements of the coating, add a right amount of bright agent, leveling agent, wetting agent and miscellaneous agent and other additives to improve the quality of the coating.
02 Diamond electroplated nickel flow
1. pretreatment before plating: diamond is often not conductive, and needs to be plated with a layer of metal through other coating processes. Chemical plating method is often used to pre-plating a layer of metal and thicken, so the quality of the chemical coating will affect the quality of the plating layer to a certain extent. Generally speaking, the content of phosphorus in the coating after chemical plating has a great impact on the quality of the coating, and the high phosphorus coating has relatively better corrosion resistance in acidic environment, the coating surface has more tumor bulge, large surface roughness and no magnetic property; the medium phosphorus coating has both corrosion resistance and wear resistance; the low phosphorus coating has relatively better conductivity.
In addition, the smaller the particle size of the diamond powder, the larger the specific surface area, when coating, easy to float in the plating solution, will produce leakage, plating, coating loose layer phenomenon, before plating, need to control the P content and coating quality, to control the conductivity and density of diamond powder to improve the powder easy to float.
2, nickel plating: at present, diamond powder plating often adopts the rolling coating method, that is, the right amount of electroplating solution is added in the bottling, a certain amount of artificial diamond powder into the electroplating solution, through the rotation of the bottle, drive the diamond powder in the bottling to roll. At the same time, the positive electrode is connected with the nickel block, and the negative electrode is connected with the artificial diamond powder. Under the action of the electric field, the nickel ions free in the plating solution form metal nickel on the surface of the artificial diamond powder. However, this method has the problems of low coating efficiency and uneven coating, so the rotating electrode method came into being.
The rotating electrode method is to rotate the cathode in diamond powder plating. This way can increase the contact area between the electrode and diamond particles, increase the uniform conductivity between the particles, improve the uneven phenomenon of coating, and improve the production efficiency of diamond nickel plating.
brief summary
As the main raw material of diamond tools, the surface modification of diamond micropowder is an important means to enhance the matrix control force and improve the service life of the tools. In order to improve the sand loading rate of diamond tools, a layer of nickel and phosphorus can usually be plated on the surface of diamond micropowder to have a certain conductivity, and then thicken the plating layer by nickel plating, and enhance the conductivity. However, it should be noted that the diamond surface itself does not have a catalytic active center, so it needs to be pretreated before the chemical plating.
reference documentation:
Liu Han. Study on the surface coating technology and quality of artificial diamond micro powder [D]. Zhongyuan Institute of Technology.
Yang Biao, Yang Jun, and Yuan Guangsheng. Study on the pretreatment process of diamond surface coating [J]. Space space standardization.
Li Jinghua. Research on the surface modification and application of artificial diamond micro powder used for wire saw [D]. Zhongyuan Institute of Technology.
Fang Lili, Zheng Lian, Wu Yanfei, et al. Chemical nickel plating process of artificial diamond surface [J]. Journal of IOL.
This article is reprinted in the superhard material network
Post time: Mar-13-2025